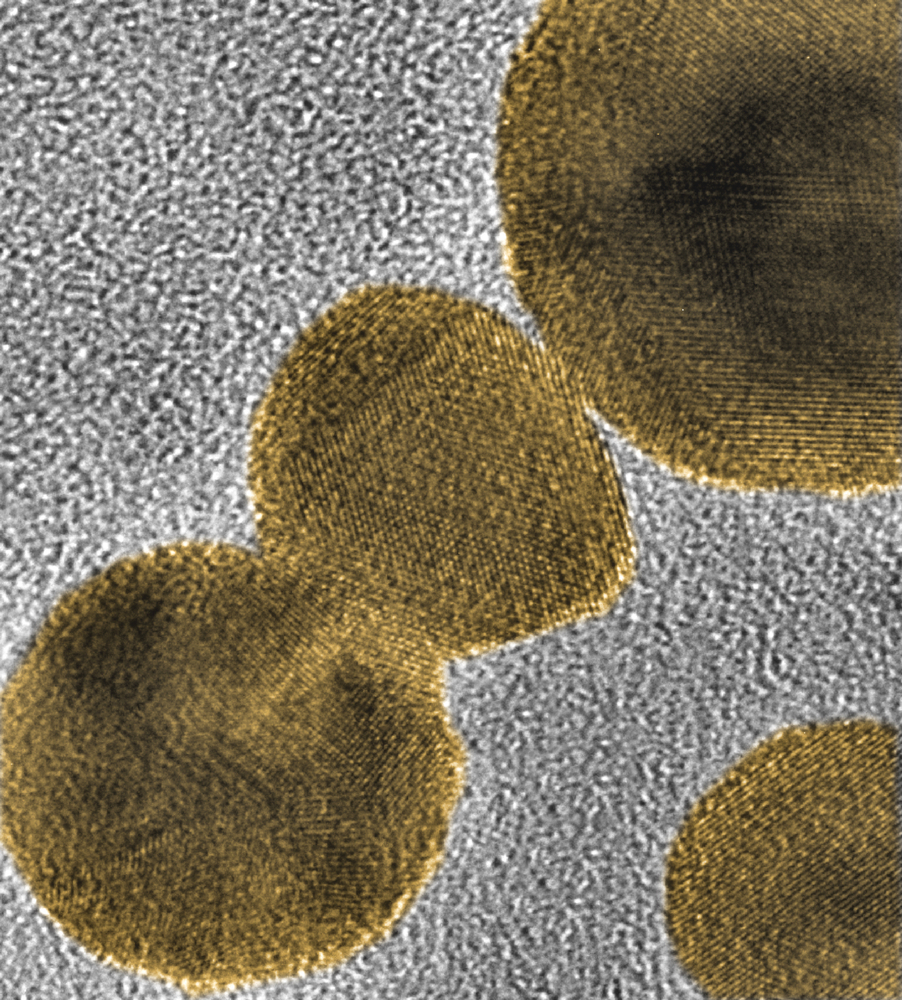
Researchers at Brown University and the University of Rhode Island (URI) at Kingston, RI, have demonstrated what promises to be a more precise method for targeting radiation therapy specifically to cancer cells. Cancer-seeking peptides ferry nanoparticles of gold to the site. The gold then helps focus radiation in a new way to increase its effectiveness in killing cancer cells.
The approach under investigation involves tethering gold nanoparticles to acid-seeking compounds called pHLIPs (pH low-insertion peptides). Cancer cells are generally more acidic than normal healthy cells, which maintain a pH of 7.4 with little variation. On the other hand, cancer cells expend substantial amounts of energy in their rapid proliferation, creating an extracellular pH level of 5.5 to 6.5 (the lower the number, the higher the acidity). pHLIPs are natural acid-seekers, which enables them to home in more precisely on the high acidity of malignant cells, thereby delivering the nanoparticle passengers straight to the cells’ proverbial doorsteps, where they then function as tiny antennas, focusing the radiation energy in the area directly around the cancer cells.
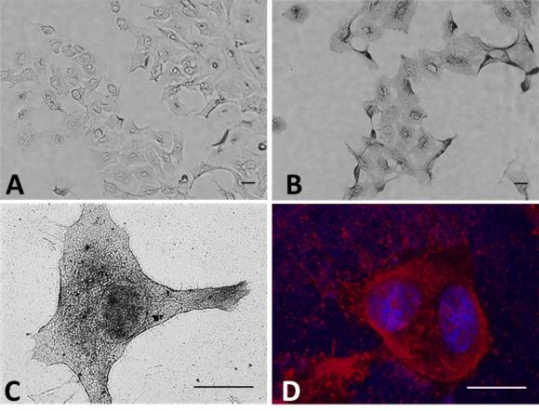
In a paper published this month in the journal Proceedings of the National Academy of Sciences, the research team explains how the novel approach substantially increases radiation therapy’s cancer-killing power in laboratory testing. The PNAS paper, entitled “Enhancement of radiation effect on cancer cells by gold-pHLIP“ (doi: 10.1073/pnas.1501628112) is coauthored by Michael P. Antosh, Dayanjali D. Wijesinghe, Samana Shrestha, Robert Lanou, Yun Hu Huang, Thomas Hasselbacher, David Fox, Nicola Neretti, Shouheng Sun, Natallia Katenka, Leon N Cooper, Oleg A. Andreev, and Yana K. Reshetnyak variously representing Brown University’s Institute for Brain and Neural Systems and Department of Physics, Department of Molecular Biology, Cell Biology and Biochemistry and Department of Chemistry and the University of Rhode Island Department of Computer Science and Statistics.
The researchers note that previous research has demonstrated gold nanoparticles’ ability to increase the effectiveness of radiation in killing cancer cells, and this improved radiation effectiveness means less radiation has to be used to achieve the desired effect, reducing adverse effects for patients, or alternatively, more cancer killing could be possible in a session using current radiation doses due to greater efficiency.
In this study, the scientists used pH Low-Insertion Peptide (pHLIP) to tether gold nanoparticles to membranes of cancer cells, which increases their effectiveness because the radiation/particle effect is very localized. They found that pHLIP significantly increases the quantity of gold particles in cancer cells, as well as the amount of cancer cell death from radiation, observing that this methodology holds promise for clinical research.
[adrotate group=”1″]
The study coauthers summarize that they found conjugation of pHLIP to gold nanoparticles increases gold uptake in cells compared with gold nanoparticles without pHLIP, with the nanoparticles distributed mostly on the cellular membranes; gold nanoparticles conjugated to pHLIP produce a statistically significant decrease in cell survival with radiation compared with cells without gold nanoparticles and cells with gold alone; and that in the context of their previous findings demonstrating efficient pHLIP-mediated delivery of gold nanoparticles to tumors, the obtained results serve as a foundation for further preclinical evaluation of dose enhancement.
 “This study was a good proof of concept, says the PNAS paper’s lead author Michael Antosh, an assistant professor (research) in Brown’s Institute for Brain and Neural Systems. “We’re encouraged by our initial results and were excited to take the next step and test this in mice.”
“This study was a good proof of concept, says the PNAS paper’s lead author Michael Antosh, an assistant professor (research) in Brown’s Institute for Brain and Neural Systems. “We’re encouraged by our initial results and were excited to take the next step and test this in mice.”
 This research is an extension of work started by husband and wife scientific collaborators Yana Reshetnyak — both biophysicists and associate professors — and Oleg Andreev, professors in the URI’s Division of Biological and Medical Physics, and professor Donald Engelman of Yale University, the inventors of pHLIP technology. The URI/Yale team had previously developed pHLIPs as a potential delivery system for cancer drugs and diagnostic agents.
This research is an extension of work started by husband and wife scientific collaborators Yana Reshetnyak — both biophysicists and associate professors — and Oleg Andreev, professors in the URI’s Division of Biological and Medical Physics, and professor Donald Engelman of Yale University, the inventors of pHLIP technology. The URI/Yale team had previously developed pHLIPs as a potential delivery system for cancer drugs and diagnostic agents.
While scientists have known about tumor acidity for years, no one had yet devised a way to target it. Dr. Engelman at Yale University’s molecular biophysics and biochemistry lab discovered the peptide that targets acidity, but had not employed it until Dr. Reshetnyak joined his lab as a postdoctoral student in 2003. She and Dr. Andreev, then a senior scientist at an anticancer drug delivery company, suggested investigating the peptides’ potential as cancer targeting agents.
peptide that targets acidity, but had not employed it until Dr. Reshetnyak joined his lab as a postdoctoral student in 2003. She and Dr. Andreev, then a senior scientist at an anticancer drug delivery company, suggested investigating the peptides’ potential as cancer targeting agents.
Drs. Reshetnyak and Andreev, both born and educated in Russia, met at a physics conference in New Orleans and have a daughter. They joined URI’s Physics Department in 2004 and established a biological and medical physics laboratory while continuing their collaboration with Dr. Engleman and their investigation of the properties of the peptide, which had come to be called the pHLIP peptide. After making some modifications, they demonstrated that pHLIP could find a tumor in a mouse and deliver imaging or therapeutic agents specifically to cancer cells.

The Yale/URI targeting system has a patent pending in the U.S. and Europe, and the researchers suggest their discovery method could also play an important role in the study of arthritis, inflammation, infection, infarction, and stroke, since those conditions also produce high acidity.
“We previously demonstrated that pHLIP-nanogold particles could find and accumulate in tumors established in mice,” Dr. Reshetnyak says. “Now our task is to test if we can treat cancer by irradiating tumors with nanogold particles more efficiently in comparison with traditional radiation treatment.”
A Brown release notes that both theoretical and experimental work had previously demonstrated that gold nanoparticles could intensify the effect of radiation due to their absorbing up to 100 times more radiation than body tissue. The radiation causes the particles to release a stream of electrons into the area around them, and if the particles are in close proximity to cancer cells, that stream of electrons would inflict damage on those cells.
 “The idea here was to bring this all together, combining the nanoparticles with the delivery system and then irradiating them to see if it had the desired effect,” says Leon Cooper, the Thomas J. Watson Sr. Professor of Science at Brown, one of the study’s co-authors, and who shared the Nobel Prize for Physics in 1972 for explaining the behavior of electrons in superconductors, and who has been working for the last several years to better understand biological responses to radiation.
“The idea here was to bring this all together, combining the nanoparticles with the delivery system and then irradiating them to see if it had the desired effect,” says Leon Cooper, the Thomas J. Watson Sr. Professor of Science at Brown, one of the study’s co-authors, and who shared the Nobel Prize for Physics in 1972 for explaining the behavior of electrons in superconductors, and who has been working for the last several years to better understand biological responses to radiation.
“Gold is an especially good choice for amplifying radiation. When matter is hit by radiation at certain energies, electrons are released through a process known as the photoelectric effect. But gold has an additional source of electron emission, known as the Auger effect, that results from the particular arrangement of electrons orbiting gold atoms. It’s the effect of the Auger electrons that the researchers were working to maximize. Working out the quantitative details of the process involved complex calculations and simulations,” Dr. Cooper explains.
Because auger electrons are low-energy, they travel only a very short distance. Indeed, their travel distance is so short that the electrons may not escape the nanoparticle if the latter is too large. Consequently the researchers had to make sure their particles were small enough to emit those electrons. The short travel distance also means that particles need to be delivered in very close proximity to the cancer cells in order to do damage, hence the need for the pHLIPs.
The scientists’ experiments showed that cancer cells irradiated in the presence of pHLIP-delivered gold had a 24-percent lower survival rate compared to those treated with radiation alone. The pHLIP samples also had a 21-percent lower survival compared to irradiation with gold only but no pHLIPs, suggesting that the pHLIPs were effective in getting the gold close enough to the cells to do damage.
The next step the researchers say will be a rodent model test of the approach, which the team intends to launch soon.
“This work is a great example of successful collaboration between Brown and URI,”Dr. Andreev observes. “We hope that the results of this research moving forward will lead to clinical application of pHLIP-based nanotechnology.”
The work was supported by the National Institutes of Health (grants 2 P20 GM103430, CA133890, and GM073857).
Sources:
Brown University
University of Rhode Island
Proceedings of the National Academy of Sciences
Yale University
Image Credits:
Brown University
University of Rhode Island
Yale University
NobelPrize.org