 Researchers at Loyola University Medical Center have begun the first clinical trial in the country designed to assess a minimally invasive treatment that can help in pain relief, treat spinal fractures and avoid new fractures derived from cancer metastasis that migrate to the spine.
Researchers at Loyola University Medical Center have begun the first clinical trial in the country designed to assess a minimally invasive treatment that can help in pain relief, treat spinal fractures and avoid new fractures derived from cancer metastasis that migrate to the spine.
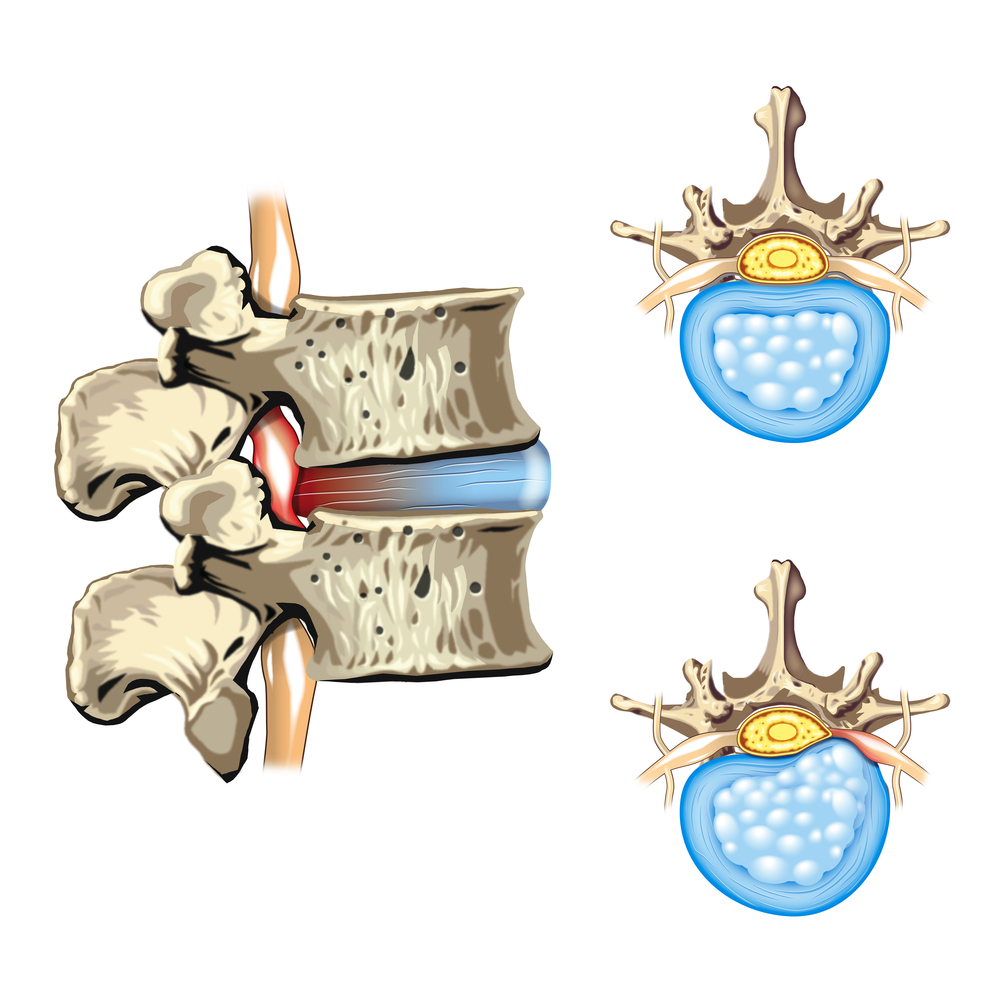
When a cancer begins its metastatic process, one of the most usual places it invades is the bone, in particular the spine. This process can render bones weaker, leading to bone damage and collapse.
Researchers are going to evaluate a treatment consisting of direct radiation delivery to the tumor to enhance spine support.
The first part of this procedure consists of a technique called intraoperative radiotherapy, a small incision directly made into the spine of the patient to insert a spinal applicator needle that can deliver radiation straight into the tumor. This technique is highly precise, allowing high doses of radiation to be delivered while reducing side effects to the surrounding healthy tissue.
In the second part of the procedure, a catheter is placed through the incision, a process called kyphoplasty, in which a small balloon at the tip can become inflated to physically support the collapsed vertebra. Moreover, a substance that can solidify is injected within the radiated area to further stabilize the spine.
[adrotate group=”1″]
In this Phase I clinical study, titled “Combining Intraoperative Radiotherapy with Kyphoplasty for Treatment of Spinal Metastases (Kypho-IORT)”, lead by William Small, Jr., MD, chair of the Department of Radiation Oncology and sponsored by the Loyola University Chicago Stritch School of Medicine, researchers will understand the positive and negative effects of putting together intraoperative radiotherapy and kyphoplasty. Factors like pain levels, pain medication usage, quality-of-life, tumor effect and associated complications will be evaluated in this study.
Patients eligible for this study must be older than 50 years of age and be diagnosed with metastatic cancer that originated from a primary solid tumor to the spine.
For further information on how to participate you can call 708-216-2568.