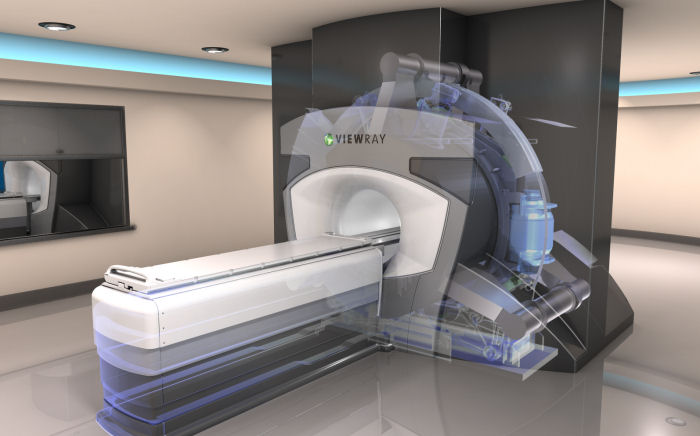
 ViewRay, a medical device company developing advanced radiation therapy technology for the treatment of cancer, has announced its proprietary MRIdian system has received CE Mark (Conformite Europeenne) approval, allowing it to be distributed throughout Europe and other regions that require the CE Mark.
ViewRay, a medical device company developing advanced radiation therapy technology for the treatment of cancer, has announced its proprietary MRIdian system has received CE Mark (Conformite Europeenne) approval, allowing it to be distributed throughout Europe and other regions that require the CE Mark.
The MRIdian radiation therapy system is the first ever on-table adaptive radiation treatment program. It is indicated for cancer care, and combines the continuous imaging technique of Magnetic Resonance Imaging (MRI), and radiation therapy.
“The European Union is the second largest radiation oncology market in the world. Receiving CE Mark is a momentous achievement and will undoubtedly fast track our conversations with medical institutions in Europe. The MRIdian system represents the most advanced radiation therapy technology available, and we are excited to partner with cancer treatment leaders to make MRI-guided radiation therapy a standard for cancer care worldwide”, Michael Brandt, senior vice president of sales at ViewRay Incorporated said in a press release.
Some of the system’s highlights include its MRI capabilities, soft-tissue monitoring, and real-time treatment planning. These technologies enable attending oncologists to implement day-to-day modifications to a patient’s prescribed regimen. Additionally, the MRIdian system reduces harmful X-ray exposure.
“Being granted CE Mark opens the door for us to deliver the world’s only clinical MRI-guided radiation therapy platform to hundreds of medical facilities,” added Chris Raanes, president and CEO of ViewRay Incorporated. “With this milestone, we look forward to making MRI-guided radiation therapy a globally accessible treatment option for cancer patients.”
[adrotate group=”1″]
In May 2012, the U.S. Food and Drug Administration (FDA) granted a 510 (k) clearance to the MRIdian system, which is currently being invested in the treatment of patients at leading centers across the United States.
“Medicine is moving towards personalized care and we’re proud to have pioneered the most customized approach to radiation delivery available,” stated the president and CEO of ViewRay, Chris A. Raanes in a company’s news release. “The MRIdian system was designed to meet the demands of adaptive treatment planning and delivery from its integrated MR imaging and high contrast soft-tissue images to its ultra-fast Monte Carlo dose calculation algorithms. It’s gratifying to see the system enable a unique clinical service that benefits patients.”