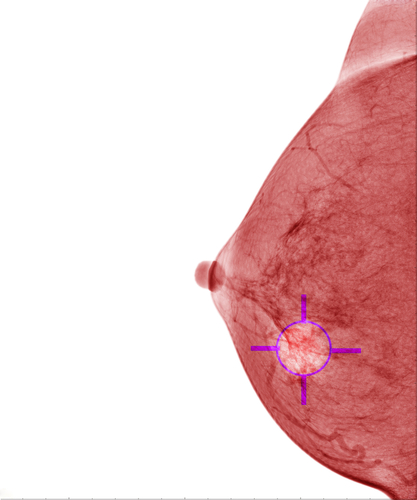
 As reported earlier by Radiation Therapy News, the National Institute for Health and Care Excellence (NICE) has approved intraoperative radiation therapy (IORT), a single dose of radiotherapy given at the same time as surgery to remove a tumor, as standard practice for patients with early stage breast cancer.
As reported earlier by Radiation Therapy News, the National Institute for Health and Care Excellence (NICE) has approved intraoperative radiation therapy (IORT), a single dose of radiotherapy given at the same time as surgery to remove a tumor, as standard practice for patients with early stage breast cancer.
NICE, an organization that provides guidance to improve health and social care in the United Kingdom, has recommended that the therapy should be offered to patients whose breast cancer is restricted to the breast and has not metastasized to the lymph nodes. Moreover, patients should be correctly informed of the pros and cons of this therapeutic approach, and doctors will be required to enter breast cancer patients treated with the IORT onto a national register, provide details of the histology at diagnosis, type, size and grade of tumor, and annotate further developments, including local recurrence and disease spread. Furthermore, they will be required to audit, review, and document clinical outcomes locally and analyze the relationship between outcomes and patients’ characteristics.
IORT is set to be offered on the British National Health System (NHS), with local NHS bodies expected to make their own funding decisions for new treatments, until a final guidance (expected to be published in November 2014) is released.
Every year, over 41,500 women and 300 men in England are diagnosed with breast cancer, the majority of which (86%) will develop early stage disease.
IORT delivers a concentrated dose of radiation therapy for 20 minutes directly to the tumor bed during surgery, helping to destroy the microscopic tumor cells that can persist after tumor removal, delivering a high dose of radiation in a single treatment session, this way preserving more healthy tissue and reducing side effects.
[adrotate group=”1″]
Usually, established radiotherapy treatments require a daily dose over a 3-week period, sometimes longer, and can be administered weeks or months after surgery or chemotherapy. However, IORT offers the potential to become a more efficient form of radiotherapy.
 “Unlike regular radiotherapy, with the Intrabeam Radiotherapy System only one dose is required. This single dose is given at the same time as surgery, eliminating the need for numerous hospital visits,” said Professor Carole Longson, director of health technology evaluation at NICE, as quoted in a recent article on OnMedica by Caroline White.
“Unlike regular radiotherapy, with the Intrabeam Radiotherapy System only one dose is required. This single dose is given at the same time as surgery, eliminating the need for numerous hospital visits,” said Professor Carole Longson, director of health technology evaluation at NICE, as quoted in a recent article on OnMedica by Caroline White.
The Appraisal Committee concluded that the data did not conclusively prove intrabeam radiotherapy to be as effective as conventional radiation therapy at preventing local breast cancer recurrence. However, it acknowledged that the recurrence rates reported in the clinical trial could be considered low in absolute terms.
“The Appraisal Committee concluded that whilst current evidence was not extensive, this type of radiotherapy was more convenient for patients and can improve a person’s quality of life,” said Prof. Longson.
“It’s still a new treatment,” Professor Longson explained. “So far, only six centres in the UK have used it to treat early breast cancer. Because it is still relatively new it is only right to recommend its use in a carefully controlled way. This will ensure patients are fully aware of the risks and benefits before choosing which treatment to have and allow doctors to gather more information about the treatment.”
So far, breast cancer charities are welcoming this recommendation. Sally Greenbrook, Senior Policy Officer at Breakthrough Breast Cancer, said: “This is great news for early breast cancer patients due for breast conserving operations.
This technique can greatly reduce the disruption, stress and inconvenience of what for some people can be over 15 additional trips to and from hospital, as well as saving the NHS money and time.”