Irving, Texas based Reata Pharmaceuticals has announced enrollment of its first patient in a Phase 2 dose-ranging study examining the safety, tolerability, and efficacy of the company’s RTA 408 Lotion (3% and 0.5%) versus vehicle for prevention and treatment of radiation dermatitis in breast cancer patients for whom radiation therapy (RT) is recommended.
PRIMROSE (A Randomized Double-Blind, Vehicle-Controlled, Parallel-Group Phase 2 Study of the Efficacy Safety, Pharmacokinetics, and Pharmacodynamics of RTA 408 Lotion in the Treatment of Patients at Risk for Radiation Dermatitis) is a multi-center study (NCT02142959) involving approximately 180 patients, with its primary efficacy endpoint being the time-averaged effect on radiation dermatitis severity.
Radiation dermatitis is a complication experienced by a majority of patients receiving radiation therapy for cancer. RT can damage the cellular structures in the skin and cause pain, ulceration, necrosis, and fibrosis of exposed skin tissues. Radiation dermatitis usually manifests within one to four weeks after initiation of RT and can result in delays in or failure to complete RT, limiting the dose effect of RT, which can negatively affect treatment outcomes. Currently there are no approved agents for the prevention of radiation-induced dermatitis. A complimentary medicine herbal remedy for RadDerm is aloe.
In an article published in Radiation Research: the official journal of the Radiation Research Society, entitled “Topical Application of the Synthetic Triterpenoid RTA 408 Protects Mice from Radiation-Induced Dermatitis,” (Radiation Research: May 2014, Vol. 181, No. 5, pp. 512-520 doi: http://dx.doi.org/10.1667/RR13578.1 Epub 2014 Apr 10) coauthors Scott A. Reisman, Chun-Yue I. Lee, Colin J. Meyer, Joel W. Proksch, and Keith W. Ward of Reata Pharmaceuticals, Inc., Irving, Texas; and Stephen T. Sonis of Biomodels, LLC, in Watertown, Massachusetts, note that free radicals produced during cancer radiotherapy often lead to dermatitis, with the degree of insult to body tissues ranging from mild erythema to moist desquamation and ulceration.
The researchers observe that this toxicity can be dose-limiting and promote chronic complications, such as fibrosis and wound recurrence. The purpose of the study reported was to evaluate if RTA 408, a synthetic triterpenoid that potently activates the antioxidative transcription factor Nrf2 and inhibits the proinflammatory transcription factor nuclear factor-kappa b (NF-kB), could protect skin from radiation-induced dermatitis.
[adrotate banner=”21″]
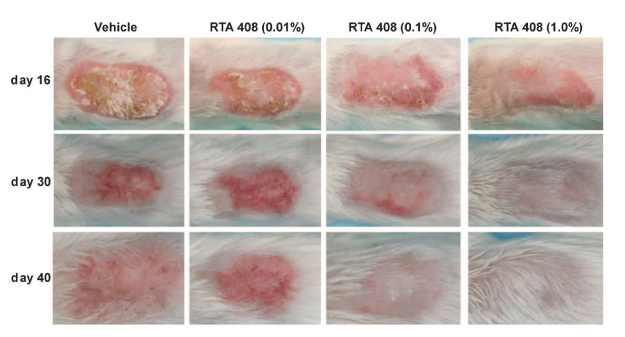
Mice were irradiated (10 Gy/day) on days 0-2 and 5-7, and RTA 408 (0.01%, 0.1% and 1.0%) was topically applied once daily starting on day 5 or up to day 40. Dermatitis severity was evaluated using a scale ranging from 0 (normal) to 5 (frank ulceration), as well as histologically. The mRNA expression of Nrf2 and NF-kB target genes in skin was also evaluated. RTA 408 (0.01%, 0.1% and 1.0%) reduced the percentage of animal-days with scores ≥ by 11%, 31% and 55% and scores ≥ by 16%, 60% and 80%, respectively.
The coauthors note that dose-dependent improvements in the appearance of skin were also manifestly visible, with RTA 408 at 1.0% eliciting a normal macroscopic appearance by the end of the treatment period on day 40, including substantial hair regrowth.
Moreover, they report that 1.0% RTA 408 markedly reduced epidermal and collagen thickening, prevented dermal necrosis and completely alleviated skin ulcers. These improvements were associated with significant increases in Nrf2 target genes and significant decreases in NF-kB target genes, concluding that together these data indicate that RTA 408 represents a potentially promising experimental therapy for the treatment of radiation-induced dermatitis.
Reata reports that RTA 408 Lotion has been assessed in a Phase 1 study which evaluated the safety, pharmacokinetics, and local pharmacodynamics of RTA 408 Lotion in 32 healthy volunteers. Twice daily application for up to 28 days of 0.5% and 3% RTA 408 Lotion was safe and well-tolerated on skin areas up to 500 cm2. No drug-related systemic adverse events were observed in any subject and systemic exposure was negligible.
According to Reata, RTA 408 Lotion is not anticipated to interfere with radiation therapy, and has demonstrated enhanced therapeutic efficacy when orally administered concomitantly with radiation therapy in mouse cancer models.
“Based on the preclinical and Phase 1 human data, we believe that RTA 408 Lotion may have the potential to become the first treatment to prevent and mitigate radiation dermatitis in breast cancer patients undergoing radiation therapy,” comments Dr. Colin Meyer, Reata’s Chief Medical Officer. “We are enthusiastic about investigating a therapy in an area where there is a lack of approved agents and high unmet medical need.”
Reata’s AIMs For Treating Radiation Dermatitis & Other Diseases
Reata Pharmaceuticals, Inc. is currently pursuing innovative research into breakthrough therapies for difficult-to-treat diseases that have significant unmet needs. The company was founded in 2002 with an understanding of the substantial technical risk involved in developing medicines, and as a result has built a business model based around small cross-functional teams, rapid evaluation of projects for biological activity and clinical viability, quick decision making, and intense analysis of scientific data to determine the direction of individual programs.
Reata’s experimental therapies, such as RTA 408, are based on its development of a novel class of drugs with potent transcriptional activity called antioxidant inflammation modulators (AIMs). AIMs are potent activators of the biological transcription factor Nrf2, which controls the body’s production of hundreds of anti oxidative and cytoprotective molecules, and is associated with protection against a broad range of diseases involving inflammation and oxidative stress. Activation of Nrf2 promotes the production of numerous antioxidative, detoxifying, and anti-inflammatory genes and moreover, inhibits NF-kB, a transcription factor that regulates many pro-inflammatory proteins.
The development of the company’s initial AIM, bardoxolone methyl, was created in partnership with Kyowa Hakko Kirin and AbbVie through deals finalized in 2009 and 2010, and the collaboration with AbbVie was expanded to the AIM platform through a deal finalized in 2011. These collaborations have led to a number of programs in the clinic today.
Reata maintains that activation of the antioxidative potential of cells within the skin prior to radiation therapy with AIM treatment could help to prevent/minimize the damage induced by radiation treatment for cancer by reducing the incidence and severity of radiation-induced dermatitis, preventing RT treatment interruptions, and reducing the long-term complications of the disease such a scarring and fibrosis. Such a treatment would provide physicians with an effective means to control a potentially dose-limiting side effect of radiation therapy, increasing the likelihood of completing the prescribed course of therapy.
Reata has completed a Phase 1 safety study of topical RTA 408 in healthy volunteers (NCT02029716), and the PRIMROSE study (NCT02142959) described above is just getting underway.
Reata says AIMs have also shown significant protective activity in models of oral mucositis induced by radiation and chemotherapy and also have potential uses in treating alopecia caused by radiation therapy and severe rash associated with anti-epidermal growth factor receptor (EGFR) therapy. The company notes that in addition to possible uses in cancer supportive care, AIM pharmacology is relevant to many other skin disorders including chronic wounds, psoriasis, atopic dermatitis, and thermal burns, and claims that AIM treatment is highly effective in a model of diabetic wound healing.
AIMs In Trials:
RTA 408 is in planning for a Phase 1b/2 trial in melanoma and lung cancer beginning in 2014 to test the ability to augment checkpoint inhibitors such as ipilimumab and PD1 inhibitors.
RTA 408 is also in planning for testing of its anti-inflammatory and bioenergetics effects in Phase 2 trials for the treatment of Freidreich’s Ataxia and Mitochondrial Myopathies during the second half of 2014.
RTA 408 has been formulated into a lotion which is being tested for use in dermatological cancer supportive care settings. The first of these is the PRIMROSE Phase 2 study to test the ability of RTA 408 to prevent radiation dermatitis described in this article.
RTA 408 has also been formulated into a sterile ophthalmic suspension and is being tested in two trials to treat post-surgical inflammation and to prevent the loss of endothelial cells due to cataract surgery.
Bardoxolone methyl (formerly RTA 402) is currently in a Phase 2 dose ranging study for pulmonary arterial hypertension.
For more information, visit:
http://www.reatapharma.com
Sources:
Reata Pharmaceuticals Inc.
Radiation Research
Image Credit:
Radiation Research


